If you turn on the stripchart you will see
that at equilibrium the concentrations are not changing, on average.
But you will see instantaneous (moment by moment)
fluctuations in the concentrations. You'll note that
the relative size of these fluctuations gets smaller as you increase
the total number of molecules in the box. This is a very general observation,
and is a fact of enormous importance, because it is utterly impractical
to do a simulation like this for the enormous numbers of molecules
that take part in any real chemical reaction. But since
the size of the fluctuations in things you can
observe macroscopically (like the concentrations)
decreases as the number of particles increases, you don't have to.
It is an empirical fact that as the number of particles increases to a very
large number the behaviour of anything observable (like the concentrations as a
function of time) becomes very simple, and depends only on other macroscopically
observable variables, like the temperature and volume of the box. This
empirical fact is essentially a statement of the existence of
thermodynamics, the science of the consequences of
hidden "degrees of freedom", such as molecules the motion of which
you cannot see.
Remarkably, deducing the existence of thermodynamics by directly considering
the limit of a simulation like this as the number of molecules is increased
to infinity is profoundly difficult, and I am not actually sure it has ever
been done. We must accept thermodynamics at present on the basis of experience
and intuition alone. (If you would like learn more about thermodynamics,
consider visiting The Second Law.)
The fluctuations have many other important roles and meanings.
I will mention just one more: the fluctuations
are (1) properties of the equilibrium system, and so can be calculated
by relatively simple theory from measurements of the system at equilibrium,
but (2) they tell you how the system
behaves dynamically when it is not at equilibrium (but fairly close).
Perhaps by comparing (in this simulation) how
fluctuations away from equilibrium happen, and
how the system approaches equilibrium -- watch the strip chart --
you will be able to see intuitively
the truth of this fundamental and important observation, which
is called the Fluctuation-Dissipation Theorem and is a bit of a trick
to prove mathematically.
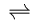 G + B
G + B