Organic Spectroscopy
Chem 203
Simple Conformational Analysis of Cyclic and Bicyclic Compounds
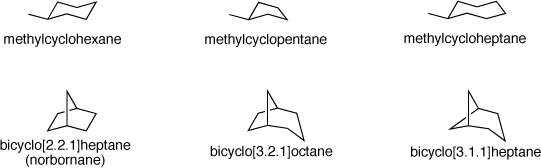
Many cyclic and bicyclic compounds can be thought of as having conformations similar to the canonical chair and boat cyclohexane structures found in cyclohexane and bicyclo[2.2.1]heptane, commonly known as norbornane. The following drawings show how low-energy conformers of cyclopentane and cycloheptane are similar to chair cyclohexane and how low-energy conformers of bicyclo[3.2.1]octane and bicyclo[3.1.1]heptane are similar to norbornane.

The images are linked to .pse files, which can be opened in PyMOL. You can download the files by clicking on the images or from the links below. If you have problems downloading by clicking on a Macintosh, hold the Option key and click with the mouse. To download on a Windows computer, use the right mouse button.
methylcyclohexane.pse methylcyclopentane.pse methylcycloheptane.pse
bicyclo[2.2.1]heptane.pse bicyclo[3.2.1]octane.pse bicyclo[3.1.1]heptane.pse
The ring systems of trans- and cis-decalin are based on canonical chair cyclohexane structures and can be thought of as archetypes for fused bicyclic systems. The images are linked to .pse files, which can be opened in PyMOL. You can download the files by clicking on the images or from the links below. If you have problems downloading by clicking on a Macintosh, hold the Option key and click with the mouse. To download on a Windows computer, use the right mouse button.
If you are having trouble downloading and viewing the .pse files, you may wish to try downloading the .pdb files, below. To download on a Macintosh, hold the Option key and click with the mouse. To download on a Windows computer, use the right mouse button.
methylcyclohexane.pdb
methylcyclopentane.pdb
methylcycloheptane.pdb
bicyclo[2.2.1]heptane.pdb
bicyclo[3.2.1]octane.pdb
bicyclo[3.1.1]heptane.pdb
trans-decalin.pdb
cis-decalin.pdb