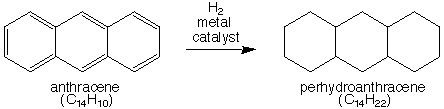
Perhydroanthracenes
Perhydroanthracene can be formed by the hydrogenation of anthracene.

There are 5 diastereomers of perhydroanthracene, which are shown below.
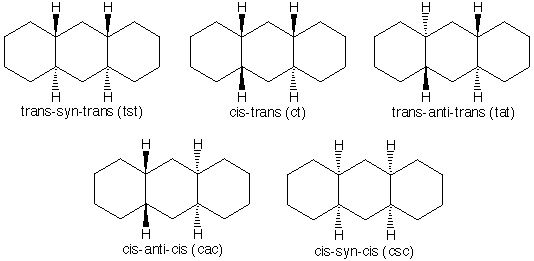
The following six molecular models correspond to the stable conformers of these five diastereomers. Note: one of these diastereomers can adopt two different conformations, both of which are shown.
Model A. What diastereomer does this model correspond to? _______
Model B. What diastereomer does this model correspond to? _______
Model C. What diastereomer does this model correspond to? _______
Model D. What diastereomer does this model correspond to? _______
Model E. What diastereomer does this model correspond to? _______
Model F. What diastereomer does this model correspond to? _______
In which of these models are all of the cyclohexane rings in chair conformations? _____________________
In which of these models is at least one of the cyclohexane rings in a boat (twist-boat) conformation? _________________
As we have discussed in class, it's important to be able to make conformationally realistic drawings of structures containing cyclohexane rings. Draw the carbon skeletons of models A-F. Do not draw the hydrogens or the C-H bonds; just draw the C-C bonds.
|
Model A |
Model B |
Model C |
|
Model D |
Model E |
Model F |
Which diastereomer is unstrained? _________
How many 1,3-diaxial interactions are present in the ct diastereomer? ________ Estimate how much higher in energy (enthalpy) it is than the unstrained one. _______
How many 1,3-diaxial interactions are present in the cac diastereomer? ________ Estimate how much higher in energy (enthalpy) it is than the unstrained one. _______
Estimate how much higher in energy (enthalpy) the tat diastereomer is than the unstrained one. _______
Explain why the two conformers of the remaining diastereomer are of roughly comparable energy. Hint: They are both very strained, but in different ways.
Two of the five diastereomers can be resolved into enantiomers. Which are they? _______________ (Hint: all conformers of each diastereomer can interconvert rapidly.)