Phosphatase Activity Modulators: Our previous work in the phosphatase enzyme inhibition area has focused on the microcystins, tautomycin, and cantharidin. The relatively recent X-ray structure of the okadaic acid-protein phosphatase I complex, in conjunction with our desire to explore new truncated structures based on the natural toxins, lead us to consider how we might truncate okadaic acid while maintaining potent inhibitory activity. The general problem is that the two most important functional groups for inhibition - the alpha-hydroxy carboxylic acid and the hydrophobic cleft-binding spiroketal - are not contiguous in okadaic acid, even though they are quite close in three-dimensional space. While it is known that the two "halves" of okadaic acid are independently active at micromolar levels, we wished to include both of these structures to maximize potency. We have thus designed "truncated" okadaic acid analogues containing a spacer group of the appropriate length that ties the two ends together in the appropriate orientation. We hypothesize that these analogues will retain significant activity, but the PP1/PP2A selectivity is difficult to predict and will be of interest in our quest to understand the structural factors that control selectivity.
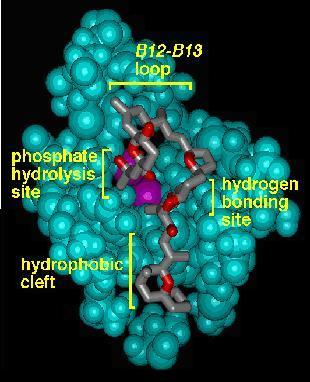
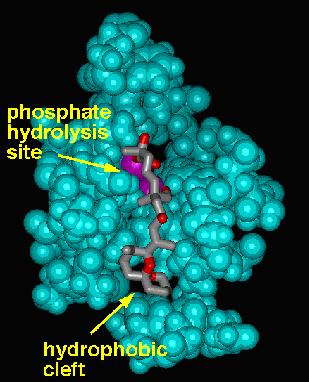
X-ray structure of OA/PP1 Model of Truncated OA/PP1
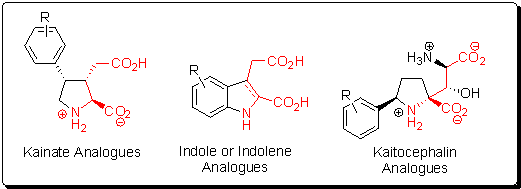
Molecular Probes for Glutamate Receptor Subtypes:
There are at least five classes of chemical receptors in
the CNS that bind L-glutamate (NMDA, KA, AMPA, ACPD, and
AP4). The binding of L-glutamate to these receptors plays a
role in such complex neuronal processes as fast synaptic
transmission, development, learning, memory, and
unfortunately, neuropathology. Because each of the
glutamate receptor classes listed above contain
inhomogeneous subclasses, new sub-type selective small
molecules need to be discovered to understand the complex
signaling of the brain. Conformationally restricted
glutamate analogues can help nail down which receptor
subtypes bind a particular glutamate conformation. We have
developed libraries based on glutamate-containing natural
products that can act as selective agonists or antagonists
for specific subclasses of glutamate receptors. This is a
very important issue because individual members of these
subtypes have been implicated in a growing number of
therapeutic areas, including stroke and neurodegenerative
diseases, cognitive impairment, epilepsy, and pain. As a
result, there is a great deal of interest in the discovery
of subtype-selective agonists and antagonists as possible
leads for therapeutic agents.
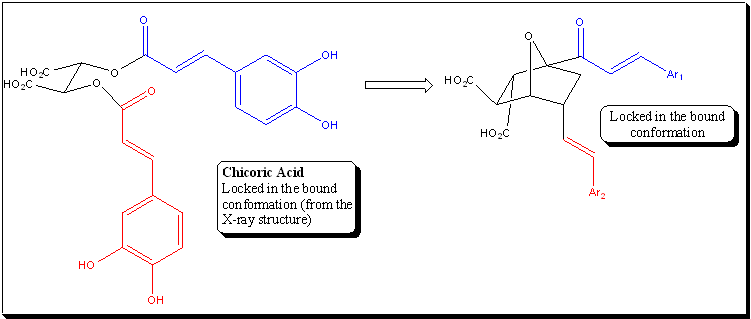
New HIV Integrase Inhibitors Based on Chicoric Acid:
L-Chicoric acid (CA) is one of the most potent and
selective inhibitors of HIV-1 integrase (HIV-IN). It
exhibits IC50 values of 145 nM for the
end-processing/strand transfer reactions and 300 nM for the
disintegration reactions, an ED50 concentration of 400 nM,
and an LD5 value of 222mM, respectively. Unfortunately,
chicoric acid itself is a rather poor drug candidate
because of its limited cell permeability, the anticipated
lability of its two ester linkages towards hydrolytic
enzymes, the potential toxicity that is generally
associated with catechol groups, of which there are two,
and the relatively large number of rotatable bonds that
might limit oral bioavailability (according to the rules
recently published by Veber). At the same time, perhaps
ironically, the very potent CA is an excellent lead
compound for optimization because there are readily
apparent potential solutions to each of these problems,
respectively: replace the carboxyl groups with precedented
surrogates (e.g., tetrazoles), substitute more robust
linkages, such as amides or ethers, for the ester groups,
identify catechol surrogates by generating and screening
libraries of CA analogues with a variety of heterocyclic
rings in place of the catechol groups, and synthesize
conformationally constrained analogues (or libraries of
analogues) with ring structures or double bonds that
prevent rotation around otherwise freely rotating single
bonds in the parent compound (preferably enforcing a
conformation closely resembling the predicted bound
conformation of CA itself, which would provide the
additional benefit of reducing the rotational entropy
penalty paid by ligands upon binding). Finally, the
relatively simple structure of CA, and the resultant
synthetic accessibility of analogues, is a further plus as
a lead compound. We are therefore "re-engineering" chicoric
acid with all of these considerations in mind.