Research
We are crafting novel chemical probes and noninvasive imaging technologies to interrogate cells in their native habitats.
Our research program bridges several disciplines and blends a variety of chemical and biological techniques. In one area, we are crafting bioluminescent probes for multi-cellular imaging in whole organisms. In a second area, we are generating chemical reactions to tag bioactive small molecules involved in cellular communication. Our third research goal involves developing tools that selectively illuminate cell-cell contacts. Collectively, these tools are enabling cellular interactions to be examined on a global scale and in whole organisms.
Developing biocompatible chemistries for imaging cellular cross-talk.
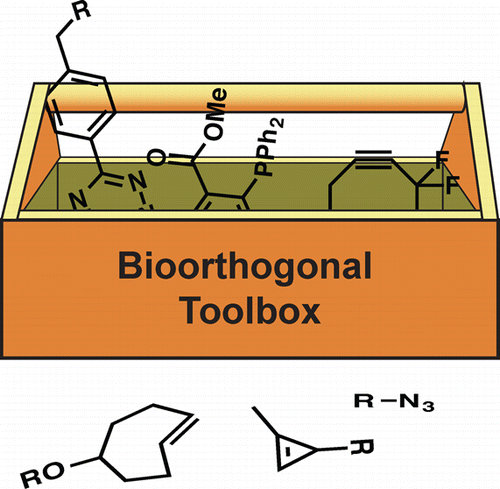
There is a need for an expanded set of chemical tools for imaging bioactive metabolites. Secreted peptides and small molecules underlie diverse forms of cellular communication, but these biomolecules cannot be easily monitored with genetic tags. We are identifying biocompatible (bioorthogonal) chemical reactions that can be used to visualize cellular metabolites in vivo. Our focus is on identifying small, stable reagents that are broadly applicable to biomolecule visualization and can be used concurrently in live cells. To date, we have developed bioorthogonal tools to profile the interplay between host cells and pathogens. We are also applying the chemical probes to track microbial peptides and lectins.
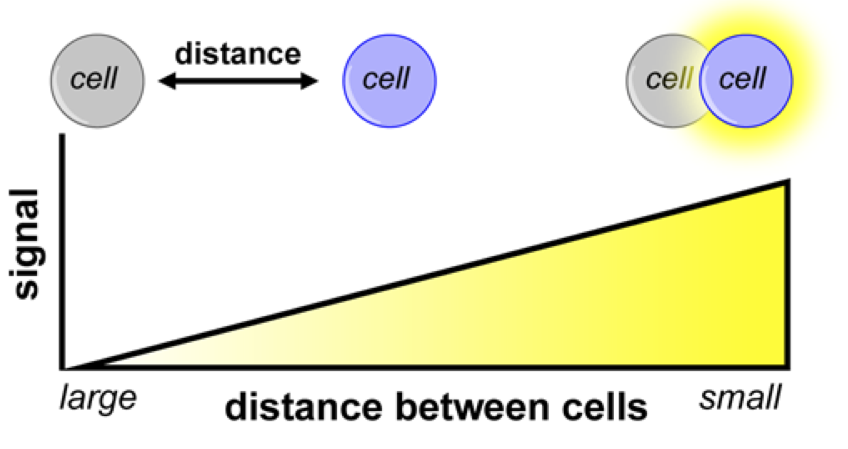
Illuminating Cell-Cell Contacts.
Target recognition and cell-cell contacts are crucial to numerous physiological processes, including neurotransmission, immune function and cell migration, yet there are few practical methods to globally assay such interactions in whole organisms. We are developing general, readily accessible methods to study cellular interactions in vivo that blend the sensitivity and noninvasive qualities of optical imaging with the spatial resolution and exquisite detail of histology. These probes are designed to produce light only when cells are in close proximity or direct contact, enabling researchers to identify cell interactions on a macroscopic scale, without the need to expose or collect tissue. We are using the contact-dependent tools to monitor tumor metastases, gut microbiota, and tissue transplants.
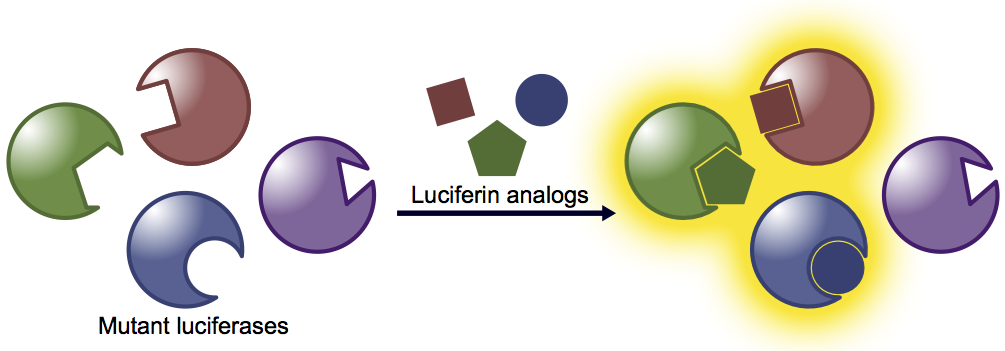
Generating orthogonal bioluminescent probes for multicellular imaging in vivo.
We are developing novel imaging tools to visualize immune surveillance mechanisms and host-microbe interactions. These processes involve multiple cell types and communication networks—features that have been historically difficult to image en masse. To capture such multicellular interactions, we are crafting designer bioluminescent probes. Our approach involves re-engineering luciferase enzymes, generating panels of mutants that accept chemically distinct luciferin analogs. When the mutants and analogs are mixed together, light is produced only when complementary enzyme-substrate partners interact. This strategy is providing a collection of bioluminescent probes suitable for visualizing multiple cell populations in vivo, analogous to the palette of fluorescent proteins available for multi-component labeling in vitro.