Tuesday, April 9, 2019
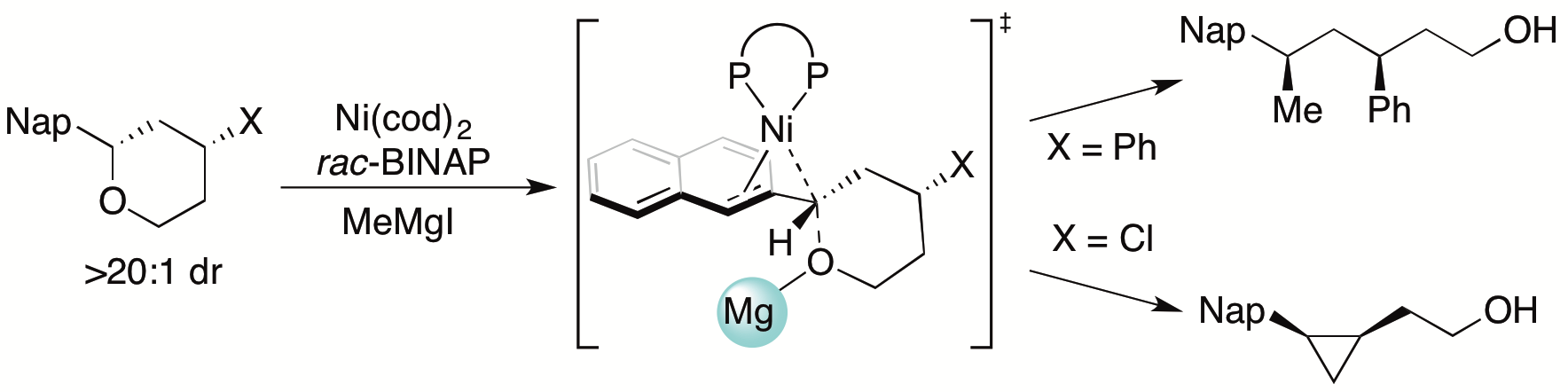
The Jarvo lab, in collaboration with the Hong lab, has completed a computational and experimental mechanistic investigation of nickel-catalyzed cross-coupling (XC) and cross-electrophile coupling (XEC) reactions of benzylic ethers. Both reactions share rate-determining stereospecific oxidative addition of the benzylic ether; after this step, the reaction mechanisms diverge with high chemoselectivity. Noteably, the XEC proceeds via a facile intramolecular substitution reaction, a distinct mechanism compared to other catalytic XEC reactions. Read the full article here.
Sub-title:
A Unified Explanation for Chemoselectivity and Stereospecificity of Ni-Catalyzed Kumada and Cross-Electrophile Coupling Reactions of Benzylic Ethers: A Combined Computational and Experimental Study
Publication:
Journal of the American Chemical Society
